Chemistry of Glass "Part 9": The two-step vitrification method.
Water evaporation, calcination, and vitrification are the primary processes used to transform a solution into glass. Between 100°C and 400°C are the temperature ranges used for the calcination stage. With the exception of the alkaline elements and some alkaline earths, it turns the majority of the elements into oxides by breaking down nitrates.
By reacting the previously acquired calcine with raw materials that primarily supply the constituents composing the vitreous network, such as silica, vitrification is carried out. According to the chemical structure of the desired glass, such processes will need temperatures around 1050°C and 1300°C. A pre-formed glass material known as glass frit often makes up the basic components.
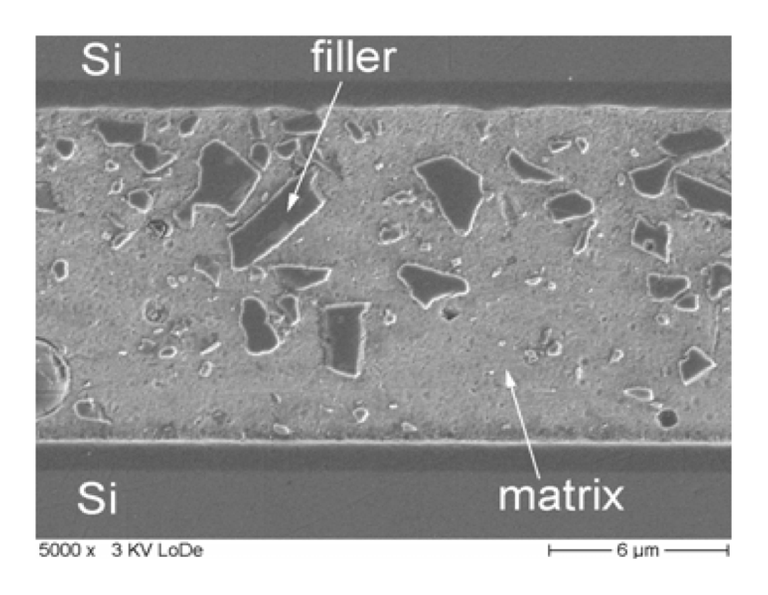
Microscopic cross sectional SEM images of glass frit bonded silicon wafers
Two phases are continually carried out in two distinct instruments: evaporation-calcination of fission product solutions and then vitrification of the calcinate created. This continuous technique was developed by the CEA to reach a performance that is suitable with the demands of the fuel plant procedures. The procedures of evaporation and partial transformation of the nitrates present in the solutions into oxides are made possible by the calcination unit. A revolving tube heated by a resistive furnace makes up this device. The revolving tube's calcinate and glass frit are fed into the vitrification furnace.
References:
- CHARACTERIZATION OF THE R7T7 LWR REFERENCE GLASS- PACAUD F.- FILLET C.- BAUDIN G. CEA Centre d'Etudes Nucléaires de la Vallée du Rhône, 30 Bagnols-sur-Ceze ^FR
- [General and inorganic chemistry book- M. Shkhashirou- H. Birqdad- Y. Qodsi- University publications. Algeria]
- Glass and Ceramic Technology Course (2021). Professor Khelifa- Department of Materials Process Engineering- University of Mostaganem. Algeria.
- Les matériaux au cœur du processus d'innovation- Clefs CEA No 59.
- Neumann, Florin. "Glass: Liquid or Solid – Science vs. an Urban Legend". Archived from the original on 9 April 2007. Retrieved 8 April 2007.
- Helène Tregouët. Structure et cristallisation de verres d’oxydes simples riches en bore et en terres rares. Chimie-Physique [physics.chem-ph]. Université Pierre et Marie Curie- Paris VI, 2016. Français. NNT: 2016PA066032. tel-01358710.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.