Chemistry of water -Part 22-
Calibration of organic nitrogen (Kjeldahl method):
After the organic nitrogen mineralization process, this method is used to titrate the organic nitrogen and ammoniacal nitrogen, then the product is passed in an alkaline medium, and the resulting ammonia is placed in boric acid. The titration of ammonia is carried out by means of an acidity meter or by the ammonium ion (NH4+) colorimetric spectrophotometer.
This method does not always allow the titration of nitrogen that is included in the composition of some industrial nitrogenous organic compounds, nor the oxidized states of nitrogen such as nitrates and nitrites, but it does allow the titration of vital nitrogen (amino acids and proteins).
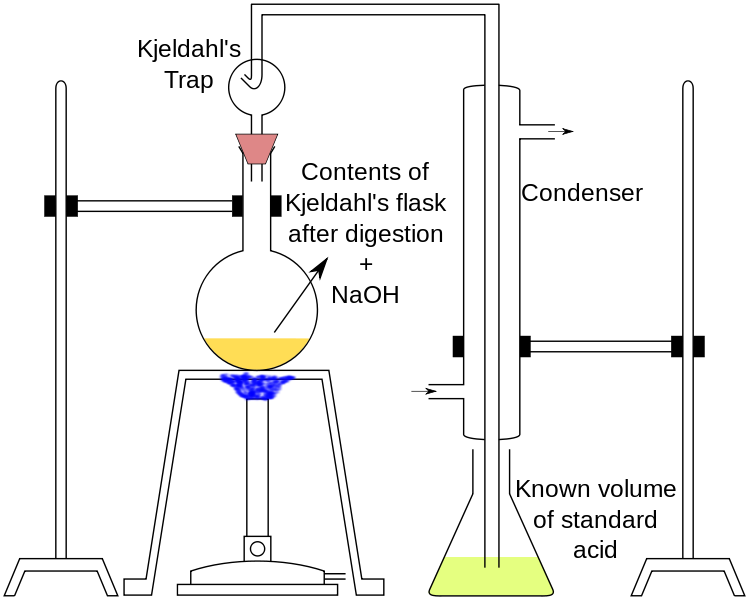
Nitrogen in the aqueous sample is concentrated by evaporation in the presence of 2 ml of sulfuric acid, and 5 ml of ferric chlorine solution prepared in hydrochloric acid is added to the flask when the evaporation process is about to end, and 5 ml of pure hydrochloric acid is added to get rid of nitrate and nitrite nitrogen, and the evaporation process continues until the sample reaches a state of almost dryness. The contents of the flask are transferred to a Kjeldahl flask, 10 ml of sulfuric acid and a drop of mercury are added, and heating is done slowly until the colour disappears or turns pale yellow, then distilled water is added after the flask has cooled.
The decanter is installed on the distillation apparatus and the volume is completed to 200 ml with distilled water. For the purpose of sedimentation of black mercury, 2 grams of sodium phosphite is added, and sodium hydroxide is added until the medium becomes alkaline and the distillation process takes place and the resulting ammonia is received in 20 ml of boric acid solution. Then the evaporation process continues until almost half of the quantity in the decanter evaporates, and a sulfuric acid solution is used to perform the calibration with the presence of the Helianthine indicator.
References:
[AQUAPROX- Livre: Traitement des eaux de refroidissement. Imprimé en France par EMD S.A.S- 53110 Lassay-les-Chateaux. N° d'imprimeur: 15566- Dépot légal: juin 2006. N° 842- Cyclus print 90°]
[Introduction to Water Chemistry (Pollution- Treatment- Analysis). Dr. Nasser Al-Hayek. Publication of the Higher Institute for Applied Sciences and Technology (HIAST). Syrian Arab Republic, 2017.]
Folco Laverdière, Anja Holstein, Laurent Thiebaut, Robert Mallee, Guillaume Gravejat, Benjamin Des clozeaux: Les principales methodes d’analyse,1999, p5.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.