PEMFC fuel cells: Improvements and Difficulties.
Massive efforts have been undertaken in the last several decades to create alternative energy sources in order to replace the conventional power sources that depend on fossil fuels. The fuel cell is a useful tool in today's strategies for reducing greenhouse gas emissions and creating alternatives to coal, natural gas, and oil-based energy sources. Invented in 1839 by Sir William Robert Grove, the fuel cell's operations were only brought to light in the 1960s by space programs that employed it to generate electricity.
Alkaline fuel cell (AFC), Direct Methanol fuel cells (DMFC), Phosphoric Acid fuel cells (PAFC), Molten Carbonate fuel cells (MCFC), Solid Oxide fuel cells (SOFC), and Proton Exchange Membrane (PEM) fuel cells are just a few of the different types of fuel cells that have been developed over the past few decades. PEM fuel cells provide advantages over other types of fuel cells in terms of system robustness, low temperature operation, high power density, fuel type adaptability, and less corrosion. Despite having all these good qualities as a source of energy, PEMFC system still confronts significant difficulties in regards to price, performance, and durability.
Proton Exchange Membrane Fuel Cell:
PEMFC (Proton Exchange Membrane Fuel Cell) is a cell having a proton exchange membrane, and it is one of the present market's five main fuel cell types which it is regarded as one of the most promising alternative clean power producers, more specifically made for automobiles and home electronics. By directly transforming the chemical energy held in hydrogen fuel into electricity with water as the only by-product, it offers the potential for zero emissions. Therefore, It is a real alternative to conventional heat engines due to its non-polluting conversion mechanism.
An electrochemical system that can transform the chemical energy found in oxygen and hydrogen into electrical energy is, in theory, what the PEMFC fuel cell is. Anode and cathode electrodes, as well as an electrolyte, are the main components of each basic cell that makes up the PEMFC fuel cell. Distributors that are either porous or have passages constructed of a graphite/polymer composite supply the anode and cathode with reactive gases.
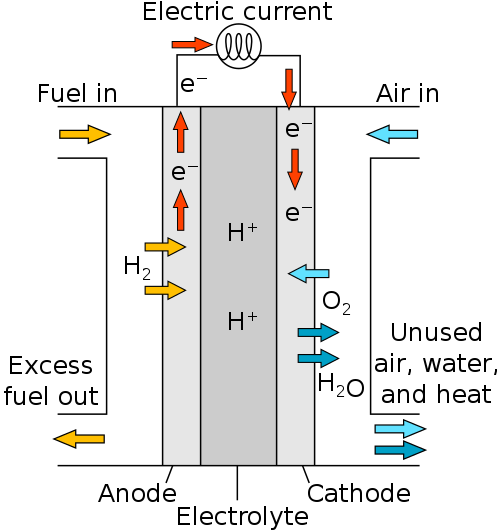
Proton Exchange Fuel Cell Diagram
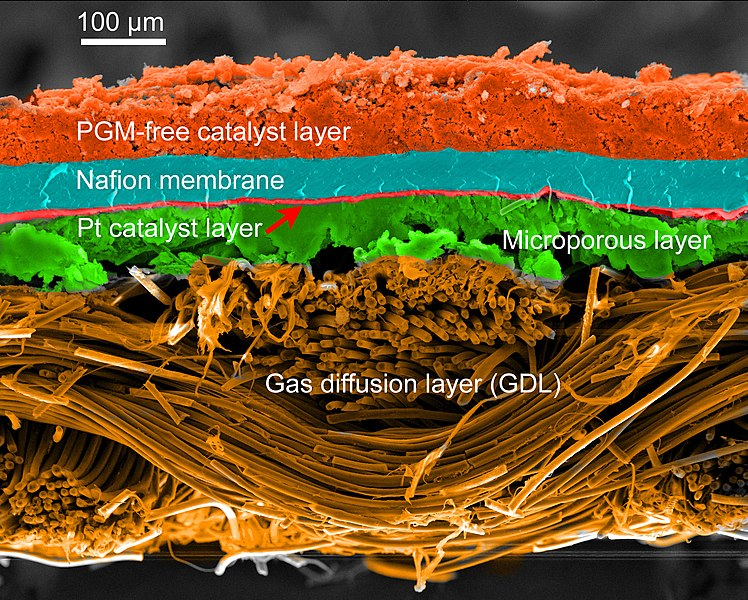
The catalyst is first placed in the form of platinum particles that are coated with the electrolyte on the surface of a carbon layer that is the active layer where the electrochemical reactions occur. The layer becomes porous after drying, permitting gases to pass through.
Finding a platinum replacement catalyst would further reduce the cost of the fuel cell, which is one of the main cost components in the system.
The transmission of homogeneous gas to the active layer, elimination of both heat and water, and capture of electrons; are ensured by the carbon fiber diffusion layer (The second layer), which provides a carbon fiber porous medium.
The polymer membrane that makes up the electrolyte prevents electrical and gaseous transfers between the anode and the cathode while also ensuring protons are transported between the two electrodes. Basically, polymers are the most effective materials, but they cannot work beyond 100°C and must be properly hydrated to perform their function as proton conductors.
The varied components' characteristics, stability over time, and technique of assembly all have a role in how effectively batteries function. The different materials utilized for the fuel cell's components must be capable of handling both charge and gaseous species exchanges. In this context, in collaboration with PSA Peugeot Citroen, the CEA has created a fuel cell that currently meets the highest standards globally for mass and size.
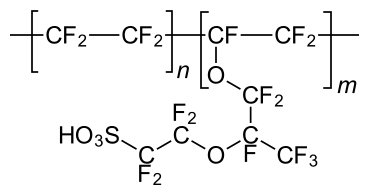
A basic solid, flexible, reasonably effective, and stable electrolyte has already been created thanks to the invention of Nafion Polymer. Nowadays, practically all of type of battery's membranes are made of polymers with a similar chemical structure. To ensure enough proton conductivity, the membranes need to be humidified using gases. However, when the temperature exceeds 80°C, a problem occurs, due to the reduction interactions between polymer chains, all of the membrane's mechanical characteristics implode at this stage.
A PEMFC fuel cell's operation is still heavily dependent on the membrane's ability to resist deterioration, because, when the membrane goes through cyclic dimensional changes that lead to rupture as the drying and hydrating stages of a cell's functioning alternate.
References:
Les matériaux au cœur du processus d'innovation- Clefs CEA No 59 – Publication: Summer 2010.
Maiyalagan T, Pasupathi S. Components for PEM Fuel Cells: An Overview. MSF 2010;657:143–89.