Natural consistent revolutions that render our water unsuitable

Large water bodies
Photo by on Nathan Dumlao Unsplash
Naturally, existence on earth would now not be feasible without the element, water.
According to scientists, one can continue to exist without taking food for some acceptable range of days but can solely proceed to be up to three days without water. This is to recognize how necessary water is to the organisms.
Water is identified to be at the pinnacle of different useful elements because almost every endeavor is being accomplished with the aid of water. Well, readers today, I would like us to learn about the natural revolutions that contaminate our natural water. So kindly loosen up and read between the lines as I take you through.
Water is any other frequent aspect regarded to man for a long time. It is one of the few elements that manifest in its pure natural form as rain due to the fact it is shaped as a result of the condensation of water vapor in the atmosphere and it is an appropriate solvent for many substances. Other natural sources of water consist of the ocean, rivers, Lake Waters, spring water, well water, and continental ice sheets.
The oceans are recognized to make up the most abundant (almost 90%) herbal supply of the earth's water resource.
But the most essential components of water sources are stated to be Freshwater which is gotten from rainfall and yet takes place naturally in rivers, lakes, spring, and continental ice sheets.
Rainfall becomes polluted when we introduce chemical compounds like nitrogen and sulfur into the atmosphere. The polluted rain will end up acidified as a result of reactions of these chemical substances with oxygen in the atmosphere. When acid rain occurs, it finds its way into the water bodies and contaminates them. Acidic rain is very dangerous and toxic.
Chemically, we can obtain drinkable pure water from impure water with the help of distillation. Distillation of impure water is one of the separating techniques employed to eliminate impurities from water. It is completed by condensing steam with the use of a Liebig condenser.
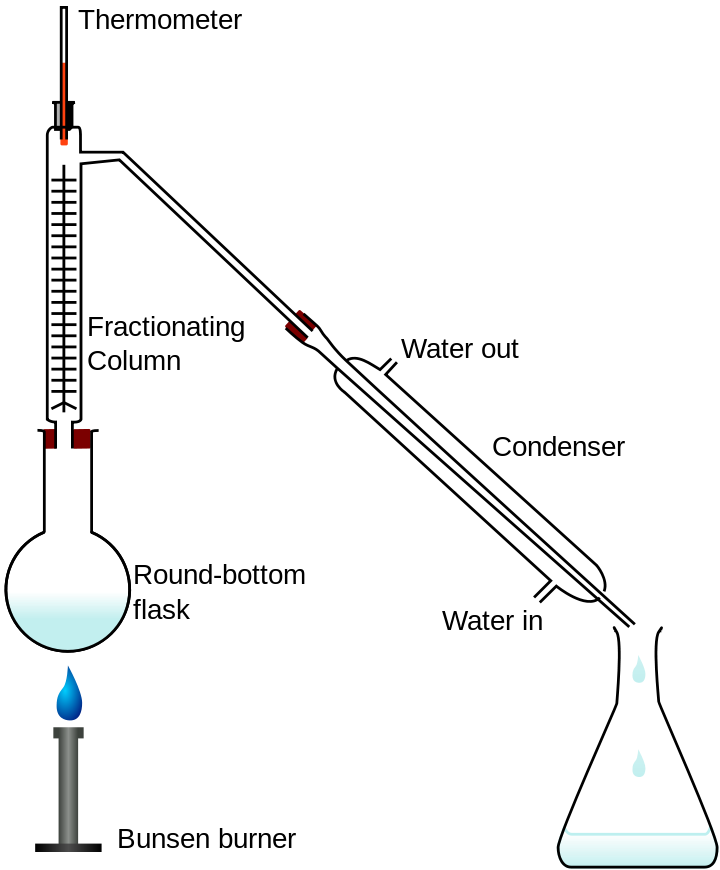
Fractional distillation lab apparatus
Source:Wikipedia, author:Theresa knott, CC BY-SA 3.0
The usefulness of a distilled water is in Regents and analytical works in special industry correctives, in the areas of drugs training and the car batteries company.
However distilled water sometimes, would no longer be fit for drinking because of its annoying taste caused by flatter and it has no flavor to improve its taste like that of tap water and bottled waters. So distilled pure water is not always a favorable choice.
Order unadulterated sources of water are the spring water, however, incorporate a huge amount of mineral salts, very tiny suspended impurities such as dirt and bacteria. But one can drink the water without any aspect effects.
However, well water which is mentioned to include a lot of clay and other mineral salts is unsafe for drinking due to underground water pollution such as pit latrine which can also contaminate the well water and for this reason renders it polluted.

Well Water
Source:Pixabay
So it is no longer reasonable to utilize well water except when it is sighted away from pit latrines and the well deepened. Deep Wells tends to be less polluted than that from the surface well but to be on the safer side, usually make sure to boil well water before drinking it.
Again, boil well water helps to take away the hardness of water received as a result of dissolved calcium hydrogen trioxocarbonate in the ground.
River water, Lake water, and seawater are additionally natural sources of water. However, one might also ask, why don't we drink from them.
Reasonably, this is because they incorporate atmospheric gases, mineral salts, bacteria, and organic remains that dissolved in them hence, making the water physique impure for human consumption.
This befell when atmospheric gases like oxygen locate their way into the water. When an extensive quantity of the gas is dissolved in these water bodies. Organic matters from homes which are delivered into the water periodically without any treatment is being decomposed by the microorganism typically and in this process, the organisms make use of the dissolved oxygen for the decomposition purpose and simultaneously, CO2 is launched into the water environment.

Water pollution
Source:Pixabay
Now, as the bacteria population advance due to too a whole lot of sewage in the water body, the oxygen degree is decreased dramatically. When it receives to the factor where the degree of oxygen dropped dangerously low, the aquatic organisms will begin to die and this will subsequently lead to the clogging up and aggravating scent from the water.
Hence the massive mass of dead undecomposed plants and animals settle to the bottom of the water body.
Besides the above justification, well water and different giant water body especially the lake, oceans occasionally do not go well with all our home motive due to the hardness of this water precipitated by dissolves rocks, soil, and some atmospheric gases they got in contact with.
The hardness of water occurs when soil particles like calcium tetraoxosulphate(vi), magnesium tetraoxosulphate(iv), and calcium hydrogentrioxocabonate(iv) dissolved in the water. When water forms lather conveniently with soap, consequently water hardness has occurred.
Hence water that acquires the above-mentioned particles will be rendered useless due to the fact that it formed disagreeable scum that sticks to the clothes, making it hard to wash.
Soaps that are manufactured with sodium octa decanete and other natural entities, when delivered to hard water would react immediately with calcium and magnesium. Insoluble scum of calcium or magnesium octadecanete would be formed. This compound produced, is what reduces the action of soup molecules and renders the soup useless as a massive quantity of soup will be required to precipitate and do away with the calcium and magnesium ions formed earlier before the washing will be completed.
This is the caus why specialists will advocate us to use detergent as an alternative of a soup due to the fact that a detergent is made with soluble calcium and magnesium salt which are soluble in water, for this reason, it will no longer form latter with tough water.
The hardness of water can be temporal or on occasion everlasting relying on the kind of salt accumulated in the water.
For instance, when bicarbonate ions of calcium, magnesium, and iron are dissolved in water, the water is said to be temporarily harden but in the presence of calcium and magnesium ions in the structure of soluble tetraoxosulphate(vi) and chloride the hardness of water turns into permanent.
After studying through the above description you will discern that natural consistent is what renders some of the water our bodies unsuitable.
Today scientists have taken advantage of hard water in the case of water grant through pipes. Water is now being supplied in a pipe made of lead with the perception that hard water does not cause lead poisoning considering that it can't dissolve in lead.
Again scientists have stated that hard water tastes better than ordinary water because of the dissolved minerals obtained in it.
Furthermore, calcium accommodates in hard water helps animals with a shell to make their shell for the reason that shells are made in most cases of calcium trioxocarbonate and to construct strong teeth and bones.
Besides this massive gain of tough water, human beings ought to make a useful aid of hard water for many different purposes. To obtain this, chemists have employed a solution that aides the softening of water hardness depending on the type of hardness in the water.
For example, temporal hard water is removed by Simple boiling. This is finalized as carbon trioxocarbonate formed can be decomposed on heating. Adding substantial quantities of slaked lime can cast off challenging water too.

Slaked lime
Source:Pixabay
While permanents are alleviated with the aid of the ability of the ion exchange method. The ion exchange medium is made up of massive polymer particles coupled to sodium counterions (a zeolite or artificial resin). When this is brought to the hard water, the exchange of ions from the hard water displaces the sodium ion from the zeolite(Na2Z) or resin (Na2R).
Sometimes caustic soda can be used for the removal of permanent hard water when the amounts of hardness in the water are no longer much. But Sodium can damage the fabric and can limit the color of animal fiber so it is not used frequently for this process.
Other negative aspects of hard water consist of stains. After washing your clothes with hard water, the scum formed via the cleaning soap would depart your garments with stain and minimize the color of the fabric material. The garments will seem Scratchy due to calcium ion.
Blockage of water flows from the pipe and showerheads are evoked due to clogging of deposits as a result of hard water.

Drop coming out of a faucet coated with calcium from the hard water
Source:Wikipedia, Author: Hustvedt, CC BY SA 3.0
Hard water cause dry and itchy skin when used for the batting. The soap residue withdraws up the leftover water on your skin thus, making your skin appears dry and itchy.
In conclusion, biological procedures that are beyond the control of Man are also one of the most common water pollutions.
This natural continuous system is what pollutes the ocean, lakes, and freshwater making it unsuitable for human consumption
Not every water physique is polluted domestically,some are natural!!
References
•Water
•well
•How To Make Well Water Drinkable And Keep It Safe To Drink
•water pollution
•Hard water
https://twitter.com/Jsalvage2/status/1331408870598717441
Nice waves and explanations !
Thank you friend.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support. Using the STEMsocial app could yield even more supporti next time.