THE CHEMISTRY BEHIND WATER TREATMENT: A THEORETICAL AND PRACTICAL APPROACH
(Edited)
Greetings and welcome back to this space of scientific dissemination, the topic that concerns us this time is associated with those chemical processes that are behind the purification of water and that are very useful to prevent the proliferation of diseases through aqueous media.

Image courtesy of: PublicDomainPictures
In this sense, it is necessary to understand that in the nature of our daily life, we are surrounded by processes where chemical systems that meet properties similar to solutions are constituted daily, so it is common to observe the formation of ions in these systems and even more so in nature where the conditions are not completely controlled.
In such a way that this type of system at the level of our nature is very common to get them, for two very obvious reasons, in the first instance water is cheap and is a liquid capable of dissolving a large number of chemicals, on the other hand, in the middle of aqueous solutions substances are in the form of ions, hence they can act in different reactions and chemical processes, in this sense, it is normal to observe aqueous processes from the depths of the sea to the interior of any living being.
So if we try to make a mental image of the process of the solutions at the molecular level, we realize that its structure is formed by particles of a substance in greater proportion to which chemically is called solvent and other particles whose quantities are lower and that we will call solute, This is distributed randomly and one envelops the other, entering into a solvation system that make possible the chemical solution, at this point, at theoretical and experimental level is expected the formation of clusters of atoms that acquire net electric charges by loss of electrons.

An example of the above is represented by oxygen dissolved in seawater, where water molecules are close to each other, while oxygen molecules are dispersed among them.
Likewise, aqueous solutions are capable of forming precipitation reactions when some dissolved salts are present, as in the example of sodium chloride in seawater; however, when the salt present is not very soluble, it tends to fall to the bottom of the place where the solution is found; this behavior of the substance is known as precipitate, the clearest proof of this behavior is represented by silver chloride, which, being not very soluble, ends up precipitating due to gravitational issues.
Based on the above, aqueous media allow the development of various chemical processes ranging from oxidation-reduction to acid-base reactions and it will be the latter where we will focus our approach to explain the treatment of water, so that the idea of acids and bases, also called alkalis, are processes that began to run in antiquity, when from certain plants began to extract alkaline substances from the ashes of the same.
Hence, in practical terms, an acid can be identified by its capacity to react with metals and carbonates, by the turning it causes on litmus paper and by its flavor. However, in chemical terms, acids are substances capable of yielding hydronium ions in the middle of an aqueous solution, as we can see in the following chemical equation, which allows us to model the behavior of hydrochloric acid when dissolved in water, generating hydronium and chloride ions.
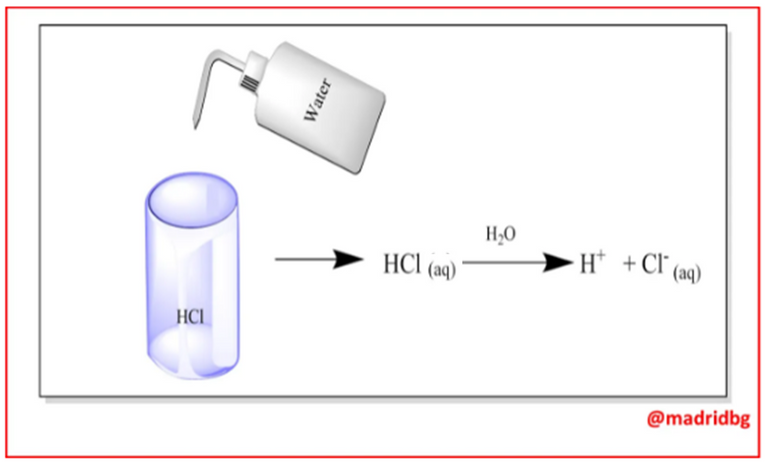
On the practical level, bases are identified by their bitter taste, they are slippery and change the color of litmus paper, on the other hand they are considered as substances capable of producing hydroxyl ions in aqueous solution, so that these substances upon contact with water dissociate completely as we can see with the case of sodium hydroxide, which produces sodium ions and hydroxyl groups when they enter into solution.
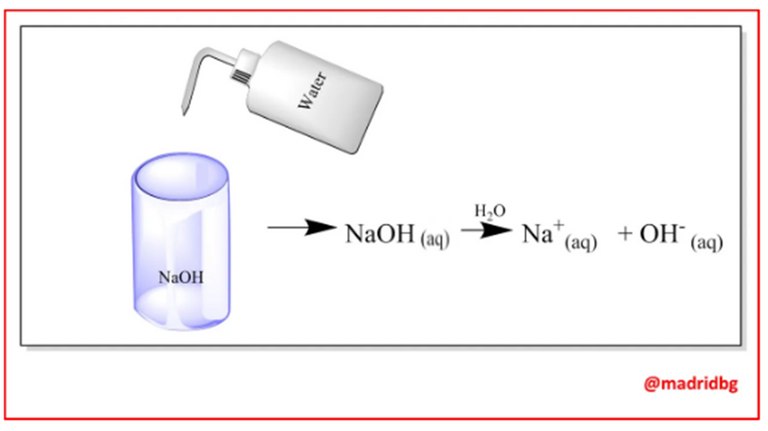
In this sense, when comparing the properties of acids and bases, we realize that one of the most striking features is their ability to neutralize the properties of one over the other, so that when they react with each other they end up generating a respective salt, a behavior that extends throughout the different chemical processes in which these substances are immersed.
Therefore and starting from the constructs described above, we have to know that water in many cases is not simply water, as it often has many impurities that make it unsuitable for certain purposes and to exemplify the above, We can establish that a chemist during his experimental processes does not use tap water to prepare the respective dissolutions of silver nitrate, since in doing so the dissolution would be covered with a milky shadow product of the reaction between the silver ions and the dissolved chlorides, thus generating the formation of silver chloride as impurities throughout the process.
On the other hand, the same tap water can be ingested without problem, so that it has been provided with another utility, now with respect to the procedure of purification of the vital liquid, this depends on the use that will be given to it or how it has been used previously, hence the water that has been used in industrial processes may require a previous treatment, the water intended for domestic use.
Likewise, water used in a chemical plant must be subjected to a more complex treatment than water that has been used at the residual level in domestic systems; however, all treatments have something in common, since during each process the aqueous chemical reactions previously studied throughout this paper will be present.
So to understand the above, let's approach the following case study: We will assume the removal of iron from drinking water coming from deep wells, according to international regulations, the recommended limit of iron present in drinking water is 0.3mg/L, however, some water wells contain a concentration of 35 mg of iron per liter of water.
Based on the above, the work methodology to eliminate excess iron consists of transforming chlorinated water into a slightly basic solution using slaked lime, chemically known as calcium hydroxide, followed by a process of oxidation of ferrous and ferric ions by the action of hypochlorite ion, and finally, the iron is precipitated in the form of ferric hydroxide from the basic solution previously prepared.
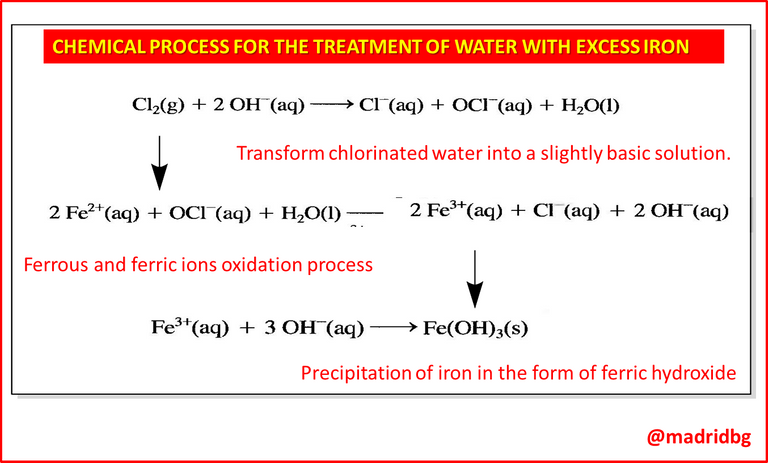
Consequently, we must bear in mind that this is only one of the methods applied and it depends on the type of pollutant or impurities present, hence it is to be expected that the processes vary, since we can apply chemical methodologies for the elimination of oxygen from thermal power plant water, elimination of phosphates from wastewater, destruction of cyanide ions in industrial operations, among others.
So chemistry offers us a wealth of possibilities in this area, hence the need to continue advancing in order to improve and control every aspect of our daily life.
BIBLIOGRAPHY CONSULTED
[1] Rincón Flórez, J. F.; Fonseca Becerra, J. E. & Carvajal Medina, R. J Calculation of thermodynamic parameters for military explosives. Application of thermodynamic fundamentals and properties of military explosives.Artículo: Acceso Online
[2] Chang, R. (2010). Química. Decima edicion. McGraw-hill Interamericana editores. ISBN: 978-607-15-0307-7.
[3] Ralph, H. Petrucci, William S. Harwood, E. Geoffrey Herring. (2003). QUIMICA GENERAL. Octava edición. PEARSON EDUCACIÓN. S.A., Madrid.
[4] WADE,LEROY. (2011). . ORGANIC CHEMISTRY. VOLUME 2. SEVENTH EDITION. PEARSON EDUCATION, MEXICO, 2011 ISBN: 978-607-32.()793-5.
OF INTEREST
1. The molecular models presented were designed by @madridbg using Chem3D and Chemdraw software.
2. For more information related to the areas of science, technology, engineering and mathematics, do not hesitate to visit #stemsocial and #stem-espanol, they are communities that promote scientific advances in these areas

0
0
0.000
Su post ha sido valorado por @ramonycajal
Gracias por el apoyo estimado equipo de #Cervantes
HI i got a few questions from reading your article and i do hope you could answer them for me.
I have completed my studie in applied sciences and i was just curious how you were drawn to some of the conclusions and information you wrote down.
The first question i have is of the second image in your article. You say here that the picture shows how oxygen is dissolved in water. However there are only water (diwaterstof mono oxide) images there and no double bond oxigens there. Where do i have to look for the oxygen is my question.
The second question is about the imagine after that. You show a picture of water being added to Hydrochloric acid, and in the reaction formula you say that this acid is in gas form. SO my question is how do you prevent in that example the fast parting of the Hydrogen and the Chloride which will cause a dangerous atmopshere. Normally, as i am thought, you would add acid to water to prevent this, But you show it the other way around. Same goes for the Sodium Chloride situation.
If you have the time to explain that to me i would be very grateful.
Greetings dear friend @dutchchemist, first of all thank you for taking the time to read my contributions and with respect to your approaches they are very good and I will try to give you a clear implication:
With respect to approach 1, notice that in the previous paragraph, I make the caveat so that we make a mental image about a dissolution and I explain that they are formed by solute and solvent, being the latter in greater proportion, so it is to be expected that in our mental image there are many water molecules that interact with each other and that is where the empty spaces in the image, would represent the few places that would occupy the oxygen dissolved in the water.
With respect to your approach 2, no doubt you are right, what I was trying to explain through the image is that both bases and acids, when they behave as strong electrolytes, tend to dissociate and the best way to represent them is through their aqueous phases. At this point, both HCl and NaOH should appear as (aq), i.e. in aqueous solution, so I must modify the respective images because it was an error on my part.
Again I appreciate you taking the time to make the respective observations.
very interesting information
!1UP
Grateful for your feedback
You have received a 1UP from @gwajnberg!
@stem-curator, @neoxag-curator, @pal-curator
And they will bring !PIZZA 🍕.
Learn more about our delegation service to earn daily rewards. Join the Cartel on Discord.
$PIZZA slices delivered:
curation-cartel tipped madridbg
@atma.love(2/15) tipped @madridbg
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.
Thank you for your constant support on my publications
Congratulations @madridbg! You have completed the following achievement on the Hive blockchain And have been rewarded with New badge(s)
Your next target is to reach 22000 upvotes.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPCheck out our last posts:
Support the HiveBuzz project. Vote for our proposal!
You may be interested in the use of Chlorine Dioxide to purify water (as used in few, but the best, water purification plants in the world, and can also used to purify the body by taking internally so the immune system can do it's job properly and reverse most disease: https://peakd.com/search?q=chlorine%20dioxide&sort=best&since=all
Some free Hive-engine tokens for you:
!PGM !PIZZA !LOL !LUV
BUY AND STAKE THE PGM TO SEND A LOT OF TOKENS!
The tokens that the command sends are: 0.1 PGM-0.1 LVL-0.1 THGAMING-0.05 DEC-15 SBT-1 STARBITS-[0.00000001 BTC (SWAP.BTC) only if you have 2500 PGM in stake or more ]
5000 PGM IN STAKE = 2x rewards!
Discord
Support the curation account @ pgm-curator with a delegation 10 HP - 50 HP - 100 HP - 500 HP - 1000 HP
Get potential votes from @ pgm-curator by paying in PGM, here is a guide
I'm a bot, if you want a hand ask @ zottone444