Soaps: An Essential and Effective Cleansing Agent |ChemFam #47|
Greetings to everyone! We have discussed the importance of drugs and chemistry of food in previous blogs. The chemistry in our everyday life related to cleansing actions and cleansing products can not be ignored from this section of discussion. So, today we shall be discussing about cleansing agents.
It is indeed very important for us to maintain cleanliness and personal hygiene to lead a healthy and disease free existence. Throughout the human evolution, mankind has sought various methods for cleansing purpose, but one such method that still stood strong is soap. For decades, soaps have played the key role in maintaining personal hygiene and cleanliness Soaps improves cleansing properties of water. They help in removal of fats which bind other materials to the fabric or skin.

The earliest recorded use of soaps traced to ancient Babylon around 2800 BCE. Those early soaps were made from combination of fats, oils and ashes. But unlike now, these soaps were used to clean textile products rather than the body. The ancient Greeks, Romans and Egyptians later embraced soaps for personal cleaning and around the Middle ages, soaps become an important specialized trade.
Chemistry of Soaps
Soaps are the detergents used since long. Soaps used for cleansing purpose are sodium or potassium salts of long chain fatty acids, e.g., stearic, oleic and palmitic acids. Soaps containing sodium salts are formed by heating fat (i.e., glyceryl ester of fatty acids) with aqueous sodium hydroxide solution. This reaction is known as saponification.
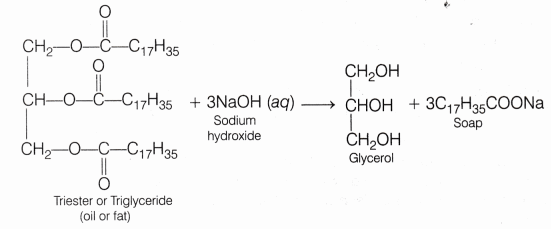
In this reaction, ester of fatty acids are hydrolysed and the soap obtained remains in colloidal form. It is precipitated from the solution by adding sodium chloride. The solution left after removing the soap contains glycerol, which can be recovered by fractional distillation. Only sodium and potassium soaps are soluble in water and are used for cleansing purposes. Generally potassium soaps are soft to the skin than sodium soaps. These can be prepared by using potassium hydroxide solution in place of sodium hydroxide.
How Soap Cleanses
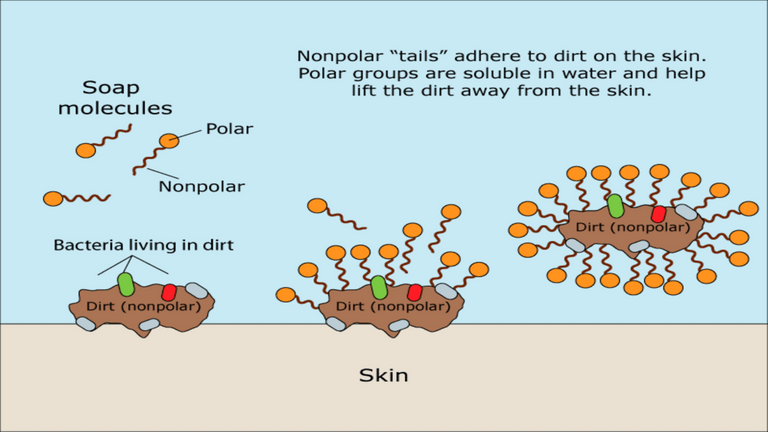
Soap's remarkable ability to cleanse lies in its amphiphilic nature, meaning it can interact with both water and oil-based substances. When soap is applied to the skin or any surface with dirt, oils, or other contaminants, its hydrophobic end attaches itself to the oily particles, while the hydrophilic end is drawn to water. As a result, soap effectively lifts away the dirt and oils from the surface, forming micelles that keep the impurities suspended in water. When rinsed off, the soap takes the impurities with it, leaving the skin or surface clean and refreshed.
Types of Soaps
Basically all soaps are made by boiling fats or oils with suitable soluble hydroxide. Variations are made by using different raw materials.
Toilet soaps are prepared by using better grades of fats and oils and care is taken to remove excess alkali. Colour and perfumes are added to make these more attractive.
Soaps that float in water are made by beating tiny air bubbles before their hardening. Transparent soaps are made by dissolving the soap in ethanol and then evaporating the excess solvent.
In medicated soaps, substances of medicinal values are added to enhance the overall properties. In some soaps, deodorants are added. Shaving soaps contain glycerol to prevent rapid drying. A gum called, rosin is added while making them. It forms sodium rosinate which lathers well. Laundry soaps contain fillers like sodium rosinate, sodium silicate, borax and sodium carbonate.

Soap chips are made by running a thin sheet of melted soap onto a cool cylinder and scrapping off the soaps in small broken pieces. Soap granules are dried miniature soap bubbles. Soap powders and scouring soaps contain some soap, a scouring agents (abrasive) such as powdered pumice or finely divided sand and builders like sodium carbonate and trisodium phosphate. Builders make the soaps act more rapidly.
Why do Soaps not work in hard water?
Hard water contains calcium and magnesium ion. These ions form insoluble calcium and magnesium soaps respectively when sodium or potassium soaps are dissolved in hard water.
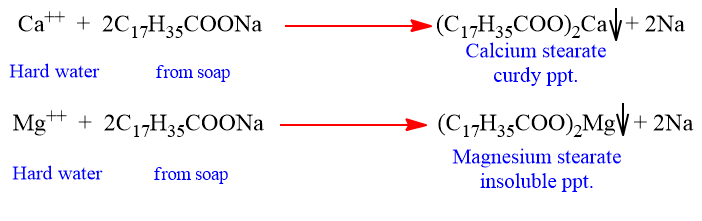
These insoluble soaps separate as scum in water and are useless as cleansing agent. In fact these are hinderance to good washing, because the precipitate adheres onto the fibre of the cloth as gummy mass. Hair washed with hard water looks dull because of this sticky precipitate. Dye does not absorb evenly on cloth washed with soap using hard water, because of these gummy mass.
Conclusive thoughts
Throughout history, soaps have proven to be indispensable in maintaining personal hygiene and cleanliness. Their unique chemical properties enable them to effectively cleanse the skin, removing dirt, oils, and harmful germs. As an essential cleansing agent, soap continues to play a vital role in promoting good health and well-being in our modern society. So, the next time you wash your hands or take a refreshing shower, take a moment to appreciate the significance of soap as a time-tested and valuable cleaning companion.
We shall meet again :)
Chemicals in Food : Debunking Myths and Ensuring Safe Consumptions |ChemFam #46|
SCRAP Giveaway | Terracore | Draw #5 |
Unveiling The Secrets of Antiseptics and Disinfectants |ChemFam #45|
What are Antimicrobials and Antimicrobial Drugs? |ChemFam #44|
Therapeutic Action of Different Classes of Drugs |ChemFam #43|
Introduction to Drugs and Drug-Target Interaction |ChemFam #42|
Scientists Analyze a Single Atom With X-Rays For The First Time |ChemFam #41|
Can We Slow Down Aging? |ChemFam #40|
Studying The Cluster Compounds: The LNCC |ChemFam #39|
Biochemistry of Calcium: Role of Calcium in Muscle Contraction |ChemFam #38|
Biosynthesis of Fatty Acids: De Novo Synthesis of Fatty Acids |ChemFam #37|
Hapticity and The Eighteen Electron Rule |ChemFam #36|
An Introduction To Organometallic Chemistry |ChemFam #35|
Applications of Zeolites: The 3D Molecular Sieves |ChemFam #34|
Properties of Zeolites: The 3D Molecular Sieves |ChemFam #33|
Zeolites: The 3D Molecular Sieves |ChemFam #32|
PS The thumbnail image is being created by me using canva.com by using template image from Ebaso


A single post with so much knowledge, a lot was learnt here.
Glad that this contributed to your vault of knowledge! Happy learning :)
Hard water makes it difficult for soap to form latter thereby reducing the strength of the soap
Lather! Yes correct, and so is the explanation for it has been given in the post itself. Happy learning!
Thank you for going through the post :)
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.
For hand washing, I've been using traditional Greek olive oil soap for a few years now, it only has like 4 ingredients and my hands don't get rashes when the weather's cold as often as they used to. And it's dirt cheap compared to more fancy soaps!
That's fantastic to hear! Traditional Greek olive oil soap is known for its simplicity and natural ingredients, which can be very beneficial for sensitive skin. The fact that you've noticed an improvement in your hands during colder weather is a great sign of its effectiveness.
Sometimes, the simplest solutions are the best. If you're happy with the results, there's no need to switch to more expensive options. Keep enjoying the benefits of your olive oil soap, and here's to healthier and softer hands! 🌿🧼