Catalyst specificity; the force converting climate killer to useful product (pt 2)
In the previous post on Catalyst specificity; the force converting climate killer to useful product , I introduced how industrial activities releases CO2 a green house gas of interest into the atmosphere. The released CO2 from industrial activities as well as other human activities that involves burning of fossil fuel trapped radiation which supposed to be reflected from the earth and thus increases the level of the earth temperature. The effect of traping the heat and subsequent increase of the earth temperature is what is referred to as global warming. I also explained how CO2 is released to the atmosphere through natural occurrence as well as the electrochemical reduction of the gas to chains of more useful products using Copper as the catalyst. In this post, i will write on why there is so much attention on CO2, transition metals as a major tool in the conversion of CO2 and how Nickel catalyst is more efficicient than Copper in the electrochemical conversion of CO2.
Why so much response on CO2 ?
.png)
Why should there be so much publications and media reports on CO2 ?. We really have other gases to be concern about which are even more dangerous in trapping heat !!!. Most of these gases in
terms of Global Warming Potentials (GWP) have more potential in heating the globe than CO2. To understand Global warming potential better, it's the absorbed heat by greenhouse gases in the space. CO2 is use as a basis for this phenomena, this means that the amount of heat absorb by a greenhouse gas is a multiple of equal amount of heat that the same mass of CO2 would have absorbed. Infact the global warming potential for CO2 is the least out of all the greenhouse gases, it's just 1 while for other gases, it depend on such gas, time frame and the method use for the data collection.
But, why do we still neglect other gases such as CH4, NO2, Water vapour and fluorinated gases and talk so much about CO2? This is because these gases are relatively less in abundance in the atmosphere in comparison to CO2 while some of them breaks down over couple of years. However, the abundance of CO2 in the space is the greatest, not only that, it also stay for thousands of years despite the fact that it's being remove massively through the process of photosynthesis and other carbon sink. Interestingly, some of these greenhouse gases when they break down produces CO2, methane is guilty of that. All these gases are emitted as a result of human activities in the industries, agricultural sector, and utilization of electronic products at home. Fluorinated gases such as Hydrofluorocarbon(HFC) and Perfluorocarbon, and others retain more energy in the atmosphere when compared to CO2. However, the amount of CO2 in the space is of a major concern to the international communities.
Although, Global warming potential of CO2 may seems to be of less concern when we compared it with the rest of the Green House Gases(GHG), but it should be noted that this gas stay longer in the space and that should be a concern to us. Since 1750 when industrial revolution started , the amount of CO2 in the space has increased from 280ppm to 415ppm which is about 45% increase. The amount of this gas we produce now will be there for generations yet unborn to deal with.
An important term of interest which gives the gas more popularity is Radiative Forcing of each of the green house gases. For the sake of clarity, Radiative force is the net difference between the amount of solar radiation absorbed by the Earth and the amount of the radiation that is reflected. So if there is positive Radiative Forcing, that simply means that the earth retain more radiation from the sunlight and if the Radiative forcing is negative the implication is that the Earth emits more radiation than it retain. The implication of latter phenomena means cooling(Life can't survive on the earth if all the radiation coming are re-emitted again,we need a blancket like these gases to warm us up but we don't need them in excess). Also, Radiative Forcing depends on the concentration of the green house gases in the space and the amount of radiation from the sunlight . It's usually quantified as W/m2 in the tropopause.
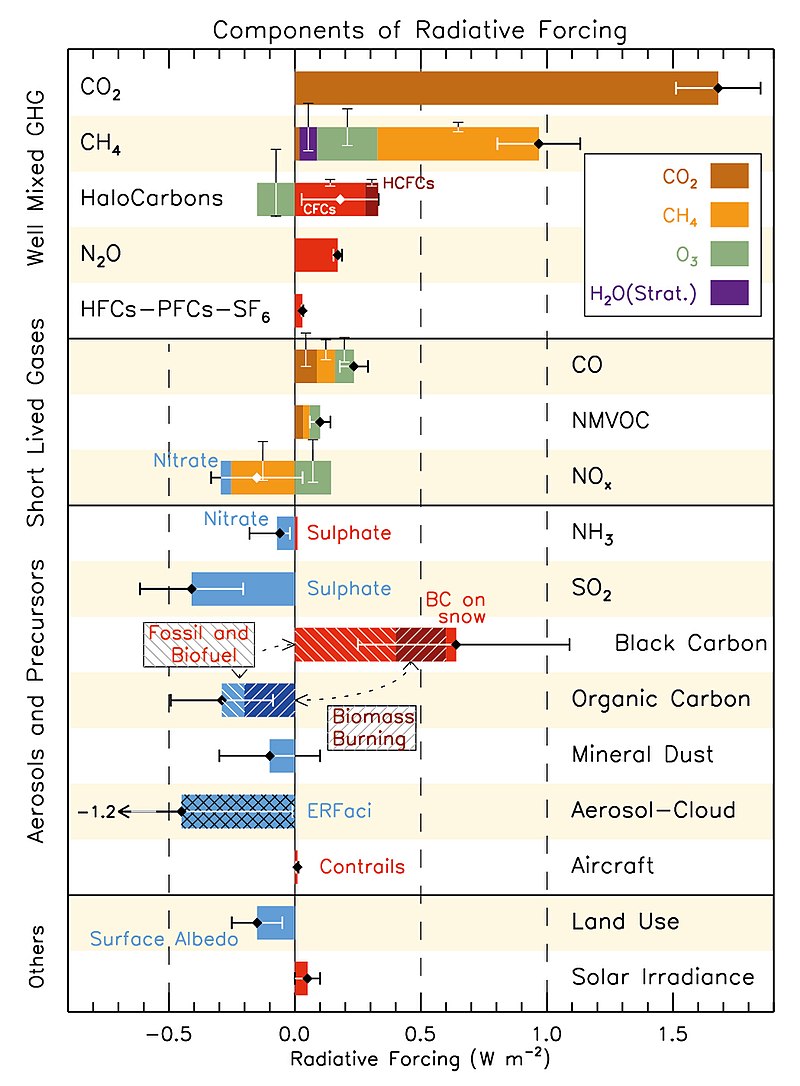
From the above chart, its quiet obvious that CO2 top the chart among well mixed green house gases even though it global warming potential is less than the rest of the gases in the same category. The implication is that it has the greatest influence in determining the balancing of energy traffic between the earth and the atmosphere. This only shows that it's an important factor that can be reckon with in terms of climate change. Study on the radiative forcing of CO2 and other green house gases which includes methane, chlorofluorocarbons (CFC), Nitrous oxide, and few other GHG between 1975 to 2018 shows an increase and dominance of CO2 in the study.
The contribution of Methane, CFC and remaining GHG to Radiative Forcing are less in comparism to CO2 in the study. The major green house gases with CO2 toping the table contributed about 96% of the radiative forcing. These gases responsible for the 96% radiative forcing are also long-lived greenhouse gases which have tremendously increased since 1750, while the short-lived greenhouse gases are accountable for the remaining 4%.
The chart below is another concern about the emission of this gas annually from different part of the world, if we keep on burning fossil fuel unabated generations unborn in the coming centuries will have a lot to deal with.
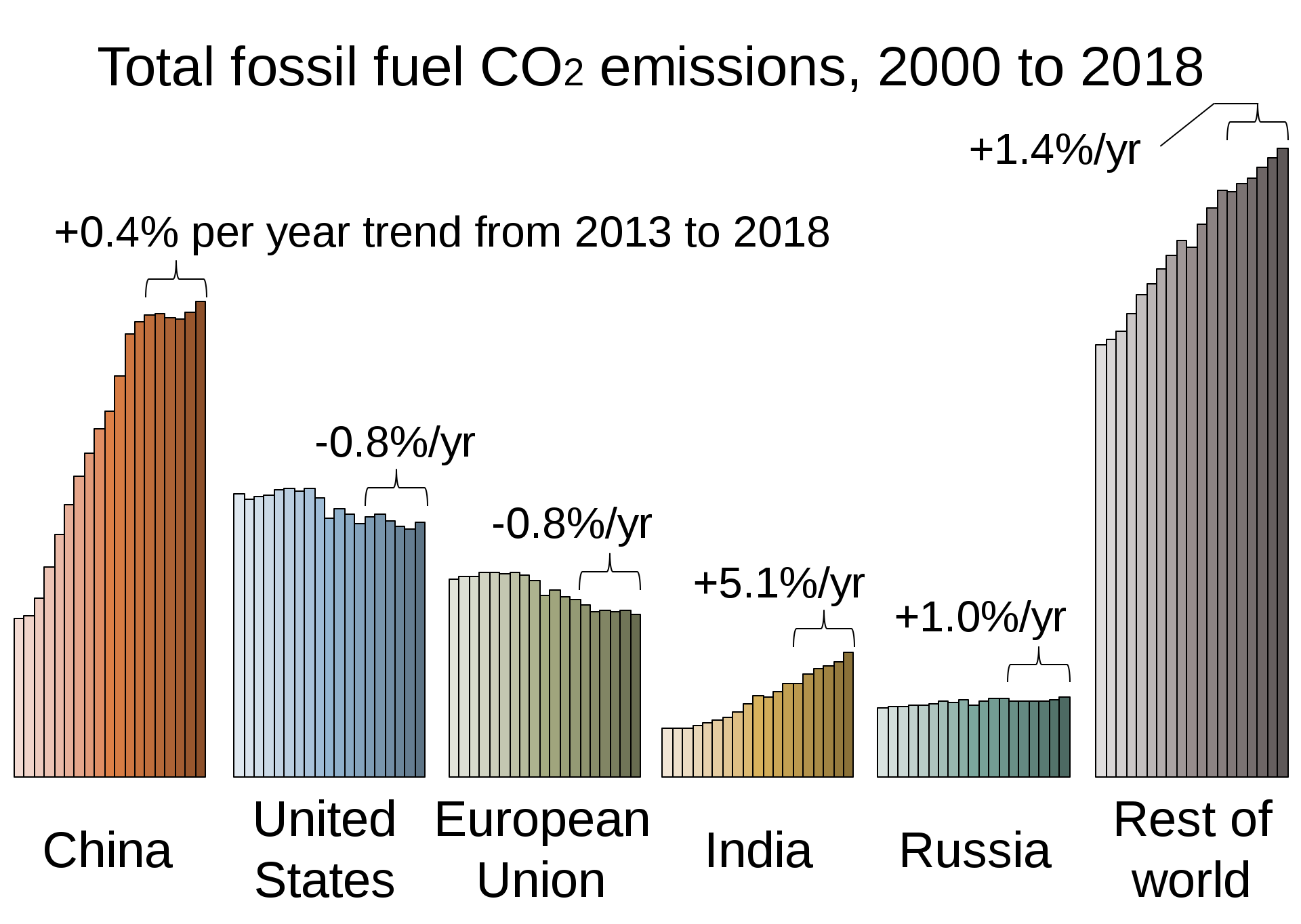
With the understanding of the terms global warming potential and Radiative Forcing its now clear why there is so much talk about CO2. With concerns raised , there is no doubt about the need for its removal.
It is expected that converting it will not only reduce the gas in the atmosphere, but that the gas will also be an alternative feedstock in the chemical industries. What makes the conversion possible is nothing else but catalysts. However, to achieve desired product, the catalyst needs to be modified so that it can be suited for the purpose.
Transition metals as Catalyst
In Catalysis, species of element/ metals used as catalysts are mainly transition metals or their compound. Transition metals are suitable for use as catalyst because they exhibit variable oxidation States which allow them to easily give or accept electrons. On the periodic table, they are part of d-block elements because of the presence of their valence electrons in the d-orbital. d-block elements means set of elements whose valence electrons is /are located in the d-orbitals. In a lay man language, orbital simply mean where electrons are located or could be found in an atom. So we have s,p,d,f orbitals with that, we have s,p,d,f block elements. All elements in s-block have their outermost electrons in the s-orbital so also with the rest.
So d-block elements have their valence electron in the d-orbital which is/are free to be exchange. We need to know that not all d-block elements are transition metals for instance, Scandium and Zinc are not transition metals because their d shell is completely filled, so they have no free electrons and therefore, they cannot have variable oxidation States.
A good example of transition metal which is being used for the conversion of CO2 to other product is Copper. It exhibit variable oxidation States such as Cu+1 and Cu+2. Because the d-orbitals of copper is incomplete, this allows the element to exchange electrons with reactants. With Cu being used as catalyst, Nickel is another d-block element which is a transition metal suitable for the work of making CO2 as an industrial feedstock.
The electronic configuration of Ni is : [Ar] 3d8 4S2. Source
We can see from here that the d-orbital is not completely filled. It has eight electrons in its d-orbitals. So Nickel has a +2 oxidation States.
Need for other catalysts to facilitate conversion of CO2 to materials rich in carbon arises because Copper has only produces materials of less number of Carbon atom in their skeleton. Copper catalyst and its alloy are only able to generate C2, C3, Ketone, alcohols, aldehyde. Some of the limitations of operations of the copper as catalyst with less include;
their poor selection of reaction which leads to large variations of products.
wastage of energy
co-production of Hydrogen of during reaction which then competes product of interest.
Improving Catalyst Activities
In other to improve the activities of Catalyst to further drive home conversion of CO2 to desired products, mechanism for conversion of the gas to methane on copper was proposed , and that mechanism requires initially reducing adsorbed carbon monoxide on the catalyst surface , this process cut off the adsorption of Hydrogen on the site of the catalyst which normally competes with the production of compound of interest. This mechanism allow HCO* which is a radical to be bounded parallel to the surface of the Cupper catalyst so that reaction will only be between Carbon and Oxygen only. This mechanism gives ample chance for transition metal to easily reduce carbon dioxide without usual Co-production of hydrogen.
This is not where it ends, stay connected for the last part which really deals with the catalyst specificity.
Meanwhile, comments and questions are welcome .
You can join stemsocial on Discord
References
https://opentextbc.ca/chemistry/chapter/12-7-catalysis/
https://www.epa.gov/ghgemissions/overview-greenhouse-gases
https://en.m.wikipedia.org/wiki/Electrocatalyst
https://en.m.wikipedia.org/wiki/Radiative_forcing
https://en.m.wikipedia.org/wiki/Greenhouse_gas
https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/3_d-Block_Elements/1b_Properties_of_Transition_Metals/Electron_Configuration_of_Transition_Metals/Oxidation_States_of_Transition_Metals#:~:text=All%20the%20other%20elements%20have%20at%20least%20two%20different%20oxidation%20states.&text=It%20was%20mentioned%20previously%20that,and%201%20unpaired%20d-electron.
https://en.m.wikipedia.org/wiki/Greenhouse_gas
https://en.m.wikipedia.org/wiki/Global_warming_potential
Greetings dear friend @steepup the science advances by leaps and bounds, your publication shows us an alternative to achieve reduce CO2 in the atmosphere, that would be a great achievement, especially if we manage to use it as industrial raw material, having an important role elements like the Copper for its conversion.
With your content I understand why, despite the existence of other greenhouse gases, dioxide is the most harmful because of its high concentrations and duration in the atmosphere, livestock annually produces large quantities of methane gas that also contributes to this effect. A pleasure to read your article allows to clarify some things about it. We continued reading us.
Thanks @amestyj for reading through. Converting CO2 into more useful products will help to sink the gas away from the atmosphere. Also gases like methane ready found usage locally, people readily use it as coking gas and it life span is short. We still have a lot to worry about seriously. Thanks again
If I understand and share with you that we have to worry, global warming has wreaked havoc on our ecosystems, these initiatives have a fundamental role to play in preventing excess greenhouse gases.
Yes, global warming has caused serious havoc on our ecosystem than we can imagine. Some countries in other to reduce burning fossil fuel has placed high tax on the generation of green house gases. That might be helpful.
@steepup : Do you mind giving references like this: [1]: https://opentextbc.ca/chemistry/chapter/12-7-catalysis/ instead of 1 Just an opinion.
Thanks @dexterdev. I tried to use an old style before not knowing that was in the past.😁😁😁. Correction on the references made.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Thanks for using the STEMsocial app and including @stemsocial as a beneficiary, which give you stronger support.